Abstract
Purpose
Since its publication in 1998, the International Prognostic Score (IPS-7) has been widely adopted as a risk stratification tool in patients with advanced stage classical Hodgkin lymphoma (cHL).1 In this study, the 5-y freedom-from-progression (FFP) ranged from 42 to 84% and 5-y overall survival (OS) from 56 to 89%. This index has demonstrated utility in the modern era, but with a significantly narrowed prognostic range.2 Further, missing factors can be problematic, limiting utility in clinical practice. A novel prognostic score (IPS-3), comprised of three of the seven IPS-7 indicators (age ≥ 45, stage IV, hemoglobin < 105), was proposed with data derived from advanced stage cHL patients enrolled on the E2496 clinical trial comparing ABVD to Stanford V.3 This model was reported to outperform the IPS-7 in predicting 5-y FFP and OS. We aimed to validate the IPS-3 model in advanced stage cHL treated with ABVD or ABVD equivalent chemotherapy in British Columbia.
Patients and Methods
The BC Cancer Lymphoid Cancer database was used to identify all advanced stage cHL patients (stage 1/2 bulky, stage 2B, stage 3/4), age ≥ 16 years, diagnosed between January 1, 1980 and June 6, 2018, treated with curative intent ABVD or an ABVD-equivalent regimen with available information for all seven IPS variables. Kaplan-Meier method and Cox proportional hazard models were used to estimate survival rates, hazard ratios (HRs), and 95% CIs. FFP was defined as time from date of diagnosis to disease progression or relapse. Log-rank testing was used to compare the survival curves between groups. As previously described3, prognostic performance and predictive accuracy of IPS-7 and IPS-3 were evaluated using concordance probability estimates (CPEs) with a Cox proportional hazard model based on risk groups.
Results
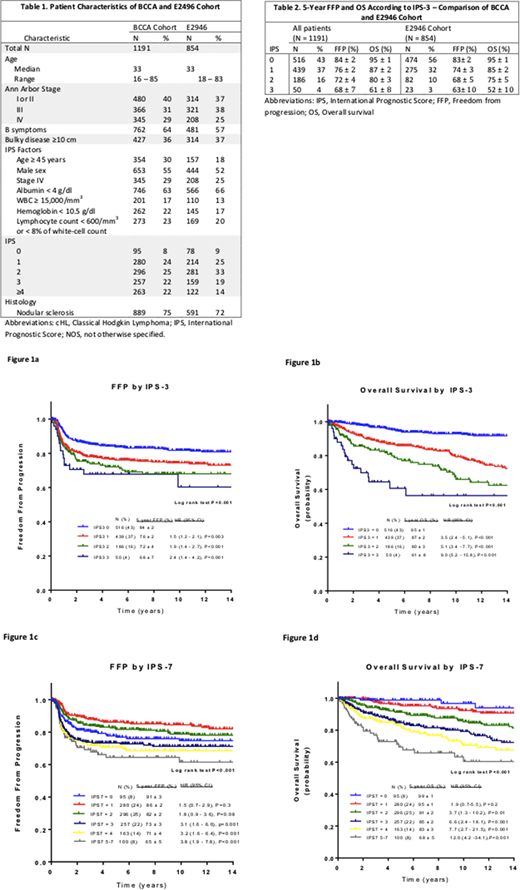
1191 patients were identified. Median age was 33 years (range 16 to 85), 30% were ≥45 years of age and 9% were >65 years of age, 55% were male and 22% had a high risk IPS-7 (≥4). Patient characteristics were similar to the E2946 cohort used to create the IPS-3 (Table 1). Furthermore, estimates of 5-y FFP and 5-y OS in the risk groups were very similar to those in the original report3 (Table 2). In the BC IPS-7 patients, 5-y FFP ranged from 65% to 91% (p<.001) and 5-y OS ranged from 68 to 99% (p<.001) and the IPS-3 model predicted a 5-y FFP of 84% ±2 for a score of 0, 76% ±2 for a score of 1 (HR 1.5, 95% CI 1.2 to 2.1), 72% ±4 for a score of 2 (HR 1.9, 95% CI 1.4 to 2.7) and 68% ±7 for a score of 3 (HR 2.4, 95% CI 1.4 to 4.2) and corresponding values for 5-y OS were 95% ±1, 87% ±2 (HR 3.5, 95% CI 2.4 to 5.1), 80% ±3 (HR 5.1 95% CI 3.4 to 7.7) and 61% ±8 (HR 9.0 95% CI 5.2 to 15.8). Restricting the analysis to patients 65 years of age and younger in our cohort (N = 1080), values for 5-y FFP were similar to the full cohort, ranging from 67 to 84% for IPS-3 and 69 to 91% for IPS-7. Values were slightly improved for OS, ranging from 70 to 95% for IPS-3 and 76 to 99% for IPS-7. Both the IPS-7 and the IPS-3 scores were not effective for predicting 5-y FFP or OS when applied to patients older than 65 years of age (all P≥0.54, N=111).
Predictive accuracy and discriminatory performance were evaluated by CPE with higher scores associated with greater accuracy. CPEs for OS were 0.63 (SE 0.014) and 0.66 (SE 0.014) for IPS-7 and IPS-3, respectively. This result may suggest a better concordance between the observed data and IPS-3; however, there was a reversal in performance when analyzing FFP, as the CPEs for FFP were 0.59 (SE 0.014) and 0.57 (SE 0.015) for IPS-7 and IPS-3 respectively.
Conclusion
This population-based study confirms that both IPS-3 and IPS-7 are prognostic in advanced stage cHL patients treated with ABVD. Unlike the original study evaluating the IPS-3, we did not find overwhelming evidence to suggest that the IPS-3 was more accurate for predicting prognosis than the IPS-7; however, given its simplicity and comparable performance to the IPS-7, IPS-3 may be more appealing for application in the clinical setting.
References:
Hasenclever D, et al: A prognostic score for advanced Hodgkin's disease. International Prognostic Factors Project on Advanced Hodgkin's Disease. N Engl J Med, 1998
Moccia AA et al: International Prognostic Score in advanced-stage Hodgkin's lymphoma: altered utility in the modern era. J Clin Oncol, 2012
Diefenbach CS et al: Evaluation of the International Prognostic Score (IPS-7) and a Simpler Prognostic Score (IPS-3) for advanced Hodgkin lymphoma in the modern era. Br J Haematol, 2015
Scott:Roche: Research Funding; Janssen: Research Funding; Celgene: Consultancy, Honoraria; NanoString: Patents & Royalties: Named Inventor on a patent licensed to NanoString Technologies, Research Funding. Sehn:TG Therapeutics: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; Celgene: Consultancy, Honoraria; Amgen: Consultancy, Honoraria; Karyopharm: Consultancy, Honoraria; Morphosys: Consultancy, Honoraria; Lundbeck: Consultancy, Honoraria; Abbvie: Consultancy, Honoraria; Seattle Genetics: Consultancy, Honoraria; Merck: Consultancy, Honoraria; Roche/Genentech: Consultancy, Honoraria. Connors:NanoString Technologies: Patents & Royalties: Named Inventor on a patent licensed to NanoString Technologies, Research Funding; Cephalon: Research Funding; Merck: Research Funding; Genentech: Research Funding; Takeda: Research Funding; Lilly: Research Funding; Roche Canada: Research Funding; Seattle Genetics: Honoraria, Research Funding; Bristol Myers-Squibb: Research Funding; Janssen: Research Funding; Amgen: Research Funding; Bayer Healthcare: Research Funding; F Hoffmann-La Roche: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal